Date: April 16, 2020
Computer simulations help to predict efficiency of novel Left Ventricular Assist Devices
The PASIM project was a Eurolab4HPC funded Business Prototyping Project by the Barcelona Supercomputing Center (BSC). An HPC business prototyping project describes the value proposition, customers, partners and business models using the business model canvas. PASIMs first milestone was to provide a regulatory numerical test to augment the data provided during pre-market approval, therefore reducing time-to-market of novel Left Ventricular Assist Devices (LVADs).
Cardiovascular diseases are one of the most critical health issues in developed countries, affecting 30% of the population. The advances in modern medicine have been mainly based on experimental research but during the last decade, in-silico experimentation has provided a powerful tool to understand physiology in health and disease and to design surgical procedures or devices.
PASIM takes profit of the extensive research on computational biomechanics done at Barcelona Supercomputing Center by transferring this technology to the users that can take the most advantage of it. Through the democratisation of supercomputing resources using cloud-based technology and reliable simulation results validated following the most strict ASME V&V40 standard, our goal is to put this tool to the device designers and clinicians fingertips.
PASIM first milestone is to provide a regulatory numerical test to augment the data provided during pre-market approval, therefore reducing time-to-market of novel Left Ventricular Assist Devices (LVADs). LVADs are implantable blood pumps that complement heart function on severe heart failure, generally used as a bridge to heart transplant. The scarce animal test done during pre-market evaluation of the LVAD tend to misrepresent the blood damage produced by the pump on human clinical usage. This regulatory numerical test allows to better predict the clot formation of a determined LVAD model, reducing patient risk and time-to-market.
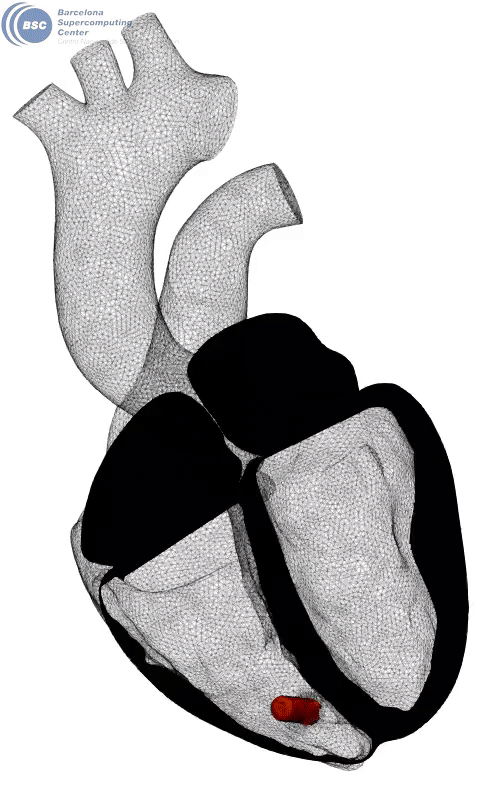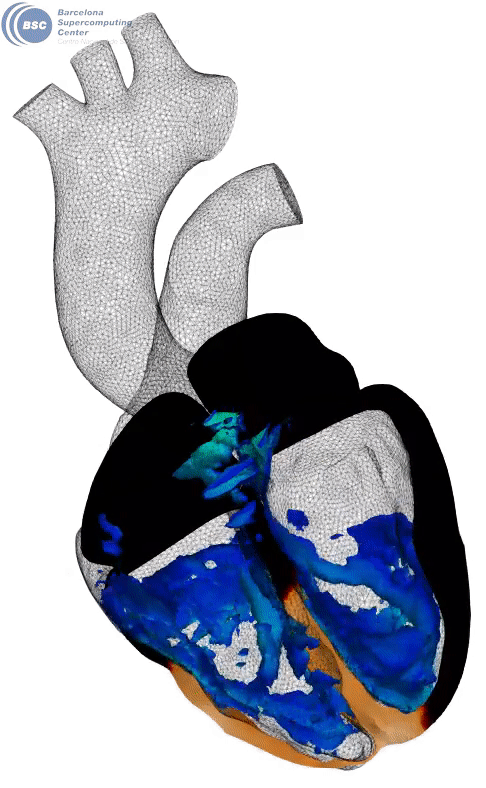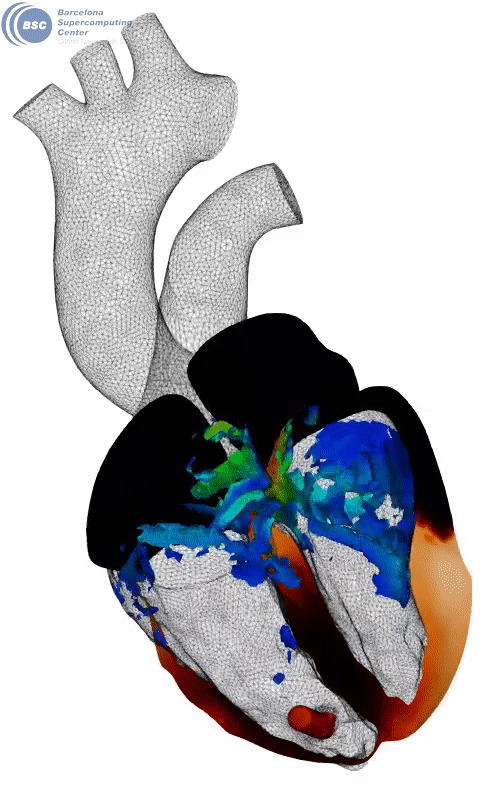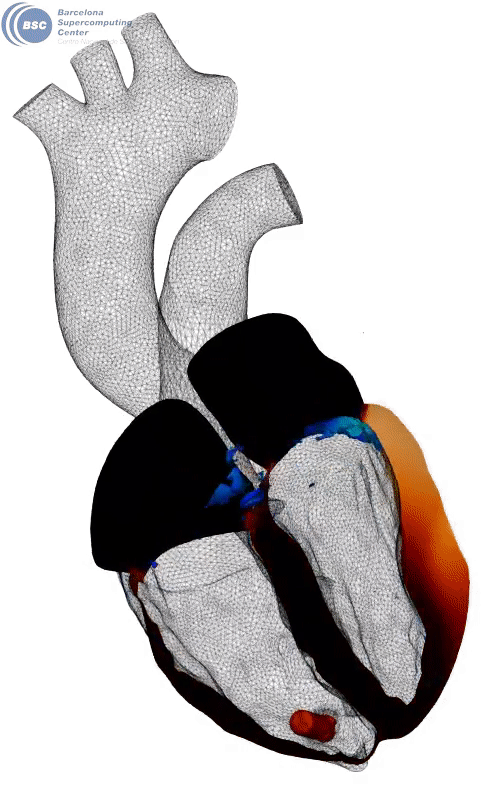
Fluid-electro-mechanical model of the human heart. The heart is suffering a III degree AV block and being paced by a leadless pacemaker in the right ventricle. You can see a slice of the myocardium, the internal fluid mesh and a leadless pacemaker implanted. The tissue is black in basal state and yellow when depolarised. Stimuli is triggered from the pacemaker on the apical region. Q-criterion (50[1/s]) showing vorticity is represented on the fluid domain.